Experiment 9 a volumetric analysis pre lab answers – Embark on a scientific journey with Experiment 9A Volumetric Analysis Pre-Lab Answers, a comprehensive guide that unlocks the intricacies of quantitative chemical analysis. Delve into the fundamental principles of volumetric analysis, exploring its significance in chemistry and its applications in various scientific disciplines.
Through a step-by-step approach, this pre-lab guide equips you with the knowledge and understanding necessary to navigate the complexities of volumetric analysis. Prepare yourself to master the art of solution preparation, glassware handling, and precise titration techniques.
Experiment Overview
This experiment aims to provide hands-on experience in the fundamental principles of volumetric analysis, a technique commonly used in chemistry to determine the concentration of unknown solutions.
Volumetric analysis plays a crucial role in various fields of science, including analytical chemistry, biochemistry, and environmental science. It enables accurate determination of the concentration of substances in solutions, which is essential for understanding chemical reactions, quality control, and environmental monitoring.
Materials and Equipment: Experiment 9 A Volumetric Analysis Pre Lab Answers

| Item Name | Quantity | Purpose |
|---|---|---|
| Burette | 1 | Measuring and dispensing known volumes of solutions |
| Pipette | 1 | Transferring accurate volumes of solutions |
| Volumetric flask | 1 | Preparing solutions of known concentrations |
| Unknown solution | 1 | Sample to be analyzed |
| Standard solution | 1 | Solution with known concentration used for titration |
| Indicator | 1 | Indicating the endpoint of the titration |
| Distilled water | As needed | Preparing solutions and rinsing glassware |
Experimental Procedure
- Prepare the standard solution by accurately measuring its volume and diluting it to a known concentration.
- Rinse the burette with distilled water and then fill it with the standard solution.
- Pipette a known volume of the unknown solution into a conical flask.
- Add a few drops of indicator to the unknown solution.
- Slowly add the standard solution from the burette to the unknown solution, swirling constantly.
- Observe the color change of the indicator to determine the endpoint of the titration.
- Record the volume of the standard solution used.
- Repeat the titration several times to obtain accurate results.
Data Analysis
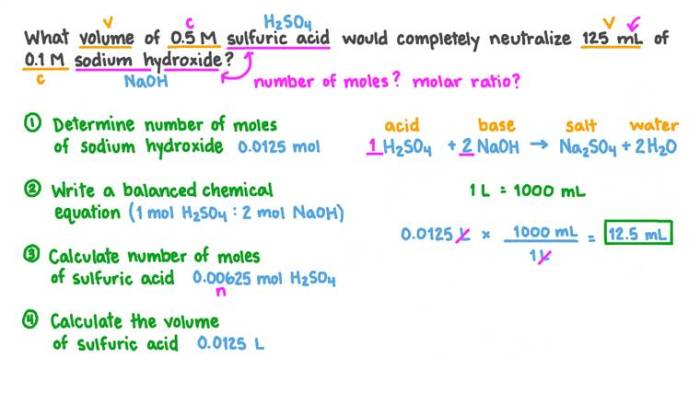
The concentration of the unknown solution can be determined using the formula:
M1V 1= M 2V 2
where:
- M 1is the concentration of the standard solution
- V 1is the volume of the standard solution used
- M 2is the concentration of the unknown solution
- V 2is the volume of the unknown solution
By rearranging the formula, the concentration of the unknown solution can be calculated as:
M2= (M 1V 1) / V 2
Potential sources of error in this experiment include inaccurate measurement of volumes, temperature variations, and indicator error. To minimize the impact of these errors, it is important to use calibrated glassware, maintain a constant temperature during the titration, and use an appropriate indicator for the specific reaction.
Discussion

The results of the experiment will provide an accurate determination of the concentration of the unknown solution. This information can be used for various purposes, such as:
- Understanding the composition of a sample
- Monitoring the progress of a chemical reaction
- Determining the purity of a substance
Volumetric analysis is a fundamental technique in chemistry that allows for precise and accurate determination of the concentration of solutions. This experiment provides a valuable opportunity to gain hands-on experience in this technique and to appreciate its importance in various fields of science.
Safety Considerations
- Wear appropriate safety gear, including gloves, lab coat, and safety glasses.
- Handle chemicals with care and avoid contact with skin and eyes.
- Dispose of chemicals properly according to laboratory regulations.
- Be cautious when using glassware and avoid sudden temperature changes.
- Keep the work area clean and free of clutter.
Illustrations and Diagrams


FAQ
What is the primary objective of Experiment 9A Volumetric Analysis?
Experiment 9A Volumetric Analysis aims to provide a thorough understanding of the principles and techniques involved in volumetric analysis, a fundamental quantitative chemical analysis method.
Why is volumetric analysis important in chemistry?
Volumetric analysis plays a crucial role in chemistry as it allows for the precise determination of the concentration of unknown solutions through accurate measurements of volume and chemical reactions.
What safety precautions should be observed during Experiment 9A Volumetric Analysis?
Proper safety precautions are paramount during Experiment 9A Volumetric Analysis. These include wearing appropriate personal protective equipment, handling chemicals with care, and following established laboratory protocols.